Relevance: Prelims: Science & Technology

Context:
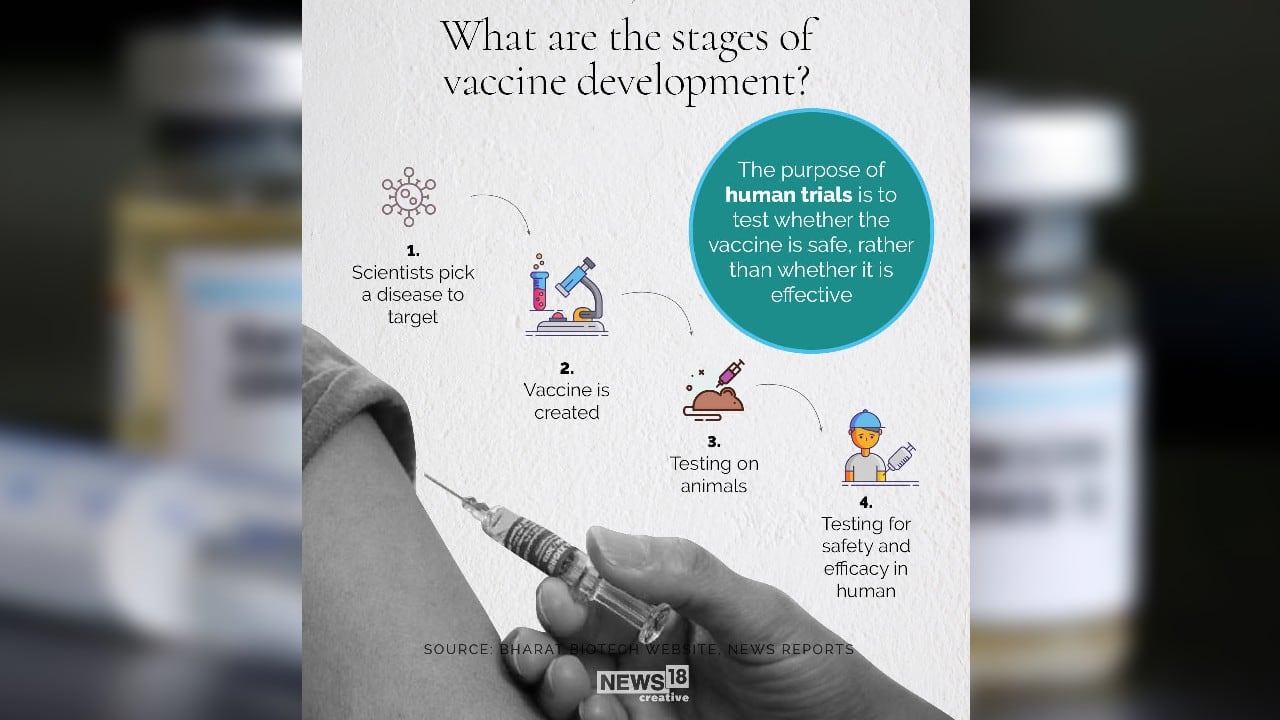
• India’s first indigenous COVID-19 vaccine (COVAXIN) developed by a Hyderabad-based company in collaboration with the ICMR is all set to be tested on humans.
• The permission from the Drugs Controller General of India to carry out phase-1 and phase-2 human clinical trials was based on the safety and efficacy results of studies on mice, rats and rabbits.
• The phase-1 trial of the candidate vaccine using inactivated (killed) novel coronavirus will begin this month to test its safety.
• The virus used for developing the vaccine was isolated by the Pune-based National Institute of Virology from samples collected in India.
• Meanwhile, a Pune-based company is all set to manufacture two-three million doses of the University of Oxford vaccine if the results of its phase-1 clinical trial are encouraging.
• Results will be expected in the first week of July.
Manufacturing:
• Millions of doses more will be manufactured if the results of the combined phase-2/3 trial are reassuring.
• In addition, the two companies are collaborating with universities and a biotechnology company to develop three more vaccines.
• With the pandemic raging and no antivirals available to treat severe COVID-19 patients, a vaccine that is even partially effective and protects for about a year will be in demand.
• Thus, an indigenous vaccine will mean guaranteed availability for Indians, while a significant percentage of the Oxford vaccine manufactured in India will be earmarked for local consumption.
• This is one reason why many countries are earnestly attempting to develop a vaccine.
• According to WHO, 17 candidate vaccines are in various stages of a human clinical trial, while 132 are in a pre-clinical trial stage.
Bypassing:
• On June 25, China’s CanSino Biologics COVID-19 vaccine, became the first off the block when it was approved for use by the military for a period of one year.
• The phase-1 and phase-2 trials found the vaccine to be safe with a “potential to protect” against the disease.
• It is unclear if the vaccination will be optional or mandatory.
• This is not the first time that countries have made vaccines under development available to the military even before the completion of the trial.
• There is growing concern that speeding up vaccine development by bypassing certain crucial stages of the trial process may prove counterproductive.
• In a poll in the U.S., one-third have said they would not get immunised against COVID-19 even if a vaccine was widely available and affordable.
• While many expect science to find a quick-fix, experts envisage 12-18 months to get a vaccine commercialised, if at all.
• But that timeline is already seen as aggressive.
• If scientists develop a safe, efficacious vaccine soon, public trust in science could grow substantially but there would be serious consequences if it fails, particularly on the safety aspect.
• Regulatory agencies have a responsibility to ensure COVID-19 vaccines deliver what they promise.
Conclusion:
• Even partially effective vaccines will be in demand, but the safety aspect is paramount.
For more such notes, Articles, News & Views Join our Telegram Channel.
Click the link below to see the details about the UPSC –Civils courses offered by Triumph IAS. https://triumphias.com/pages-all-courses.php


