Relevance: Mains: G.S paper II: Governance: health
Context:
- As ‘Coronavirus’ or 2019 n-CoV spreads rapidly across the world, it has given rise to apprehensions over whether the epidemic will impact the supply of active pharmaceutical ingredients (API) from China (the largest producer of APIs) to India and other countries.
Cautions in India’s pharma industry:
- India’s pharma industry is already worried.
• It has hinted that a rise in medicine costs cannot be ruled out even as inventories are comfortable for three months.
• The 2019 n-CoV cannot travel through consignments, as the virus can survive for just four or five days in the open.
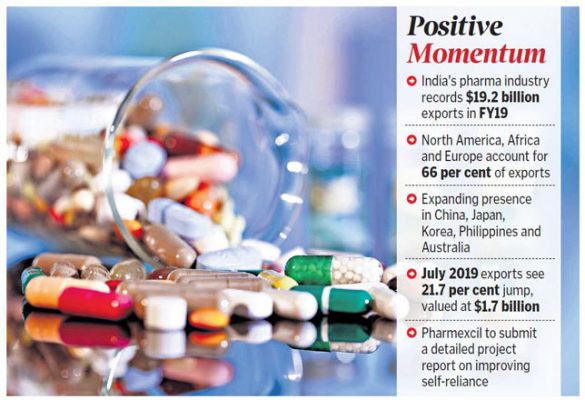
• India imports 68-69 per cent of its API requirements from China, which amounts to $2.4 billion.
• These imports are used to manufacture key medicines such as paracetamol, metformin, ofloxacin, metronidazole, ampicillin and amoxycillin.
• Supply disruptions have occurred in the past — during the 2008 Beijing Olympics, for instance, these units were shut down over environmental concerns. A similar cutback took place in 2018.
• It is noteworthy that these shocks, in fact, boost India’s API exports, which account for about a fourth of the country’s total pharma exports of $20 billion.
Self sufficiency needed in India’s pharma sector:
- The Centre has rightly acknowledged the need for self-sufficiency in APIs, setting up an ‘inter-departmental’ task force in 2018 to look into the issue.
• It is another matter that earlier committees have delved into the issue of boosting API output.
• China’s cost of production is way cheaper than India’s; even so, domestic output must be regarded as a strategic prerogative.
• It will also enhance India’s negotiating capacity in future trade engagements with China. The public sector may have to take the lead here, while raising existing R&D incentives for the private players.
• It is unfortunate that the Budget overlooked this aspect.
• The now-struggling Indian Drugs and Pharmaceuticals Ltd can be roped in for producing essential APIs.
• Its role in ramping up the production of tetracycline during the 1994 plague epidemic is instructive in this regard.
Way forward:
- In 2015, the ‘Katoch committee’ put out a set of recommendations on reviving API production, which include reviving PSUs for manufacturing critical drugs such as penicillin and paracetamol.
• The setting up of mega parks with common effluent treatment plants, testing facilities and captive power plants has been mooted.
• On the financial side, the panel has suggested setting up a professionally managed equity fund for producing APIs and duty exemptions for capital goods imports.
• These facilities can developed to world class standards, restoring the recent damage to India’s reputation on the quality front.